
Lab Website:
Email:
Office:
Phone:
Administrative Assistant:
Courses:
Research-at-a-glance:
Affiliations:
Biography:
John Essigmann is the William R. (1956) and Betsy P. Leitch Professor in Residence of Chemistry in the MIT Department of Chemistry and Professor of Toxicology and Biological Engineering in the MIT Department of Biological Engineering. He was the Associate Head of the Department of Chemistry until 2012, responsible for graduate and undergraduate education, and since that time he has been the Director of the MIT Center for Environmental Health Sciences.
Professor Essigmann was brought up in Medford, MA, a suburb of Boston and is a lifelong resident of the Boston area. He went to Northeastern University for his undergraduate degree in chemistry and subsequently earned his Ph.D. from MIT with Professor Gerald Wogan, a pioneer in the field of toxicology.
Research:
The research objective of the Essigmann laboratory is to understand the relationship between the structures of lesions formed in the genome by DNA damaging agents and the specific biological endpoints of mutation, cancer, and cell death. In the area of carcinogenesis, they probe the molecular etiology of human cancer. Their parallel studies on antitumor drugs focus upon uncovering the mechanism of action of existing drugs. Based upon that understanding, the Essigmann laboratory design novel compounds that could be useful for the treatment of cancer.
The work of this laboratory addresses the biochemical mechanisms by which cells respond to specific forms of DNA damage. The rationale of our work stems from the possibility that the DNA adducts caused by DNA damaging agents will be either mutagenic or cytotoxic, or both
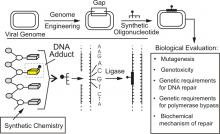
One thrust of the Essigmann lab is to study the mutagenic effects of DNA adducts at a specific site. Exposure of cells to DNA damaging agents usually results in the formation of a vast population of structurally heterogeneous carcinogen-DNA adducts. It is hypothesized that misreplication of adducts initiates cells along the pathway to malignancy. A large body of evidence indicates, however, that only a subset of the adduct population is likely to contribute to mutagenicity and carcinogenicity. Orignially, a central problem in the field of carcinogenesis was the lack of an experimental system to identify which DNA lesion gave rise to which types of mutations. The Essigmann laboratory established such a system.
As shown in this figure, the genome of a virus or plasmid is processed by using recombinant DNA techniques to situate a small gap at a specific site. An oligonucleotide containing a single DNA adduct is then synthesized and ligated into the gap. The site specifically modified genome is introduced into a bacterial or mammalian cell, allowed to replicate intra- or extra-chromosomally and, finally, progeny are isolated. Reduction in the yield of progeny is an indication of the genotoxicity of the adduct. We also determine the type, amount, and genetic requirements for mutagenesis induced by the adduct. This technology enables one to rank the mutagenic and genotoxic potentials of the various adducts that form in the genomes of cells treated with DNA damaging agents. Our future studies will be in three areas: (i) understanding how aflatoxin B1 (AFB1) causes mutations that give rise to tumors, (ii) understanding how oxidized DNA bases give rise to mutations and (iii) trying to apply knowledge of mutagenesis toward the development of safer drugs.
In addition, the Essigmann lab focuses on the design of programmable antitumor drugs through Fatal Engineering. It is a goal of cancer chemotherapy to achieve the selective killing of tumor cells while minimizing toxicity to normal tissues. The lab’s studies on cisplatin suggested a new approach toward the design of anticancer drugs. Specifically, they decided to synthesize a DNA binding molecule that will form adducts that, if not repaired, would inhibit DNA replication and transcription. To accomplish this objective, the Essigmann lab would exploit the abundance of known tumor specific proteins to bias repair so that it would occur in normal cells and fail in tumor cells, thus making the latter preferentially succumb to the drug.
One of the attractive features of this approach toward therapeutics is the fact that the toxin can be re-programmed with ligands that attract other tumor specific proteins. They have begun to prepare molecules that form DNA adducts attractive to the androgen receptor, which is over expressed in prostate cancer cells. This overall strategy could be generalized to combat a wide range of cancers and possibly even viral diseases.


